Past Issues
Effect of Different Parts of Acacia Nilotica on Serum Testosterone Levels in Male Rabbits
Syed Muhammad Imran Shah1, Ishrat Younus1*, Laila Anwer1, Faiza Fakhar1 and Farzana Sadaf1
1Department of Pharmacology, Faculty of Pharmacy, Hamdard University, Karachi.
Corresponding author: Ishrat Younus, Department of Pharmacology, Faculty of Pharmacy, Hamdard University, Karachi. Email: [email protected]
Received: August 01, 2021
Published: December 29, 2021
ABSTRACT
Background and Objectives: Herbal contraceptives are used in Africa and Pacific Asia including China, India, Pakistan and Bangladesh but scientific data on their safety and efficacy are not well established. This study was aimed to evaluate the effect of different parts of Acacia nilotica on serum testosterone levels in adult male rabbits (Oryctolagus cuniculus). Material and Methods: Forty-five sexually mature male rabbits were grouped in nine groups, having five rabbits in each group. Rabbits of Group I (b), I (f), I (l) and I (p) were administered prepared Aqueous extracts of Acacia nilotica bark, flower, leaves and pods at daily oral doses of 400 mg kg-1 of body weight, while rabbits in Group II (b), II (f), II (l) and II (p) were given daily oral doses of 400 mg kg-1 of 75% Ethanolic bark, flower, leaves and pods extracts of Acacia nilotica, up to eight weeks. Serum testosterone levels were tested following baseline, a second, fourth, sixth and eighth week of treatment. Results: Significant decrease in the serum testosterone levels was seen in Group I (p) in which the rabbits were administered an aqueous extract of Acacia nilotica pods (p < 0.05). Conclusion: It is concluded that administration of 400 mg kg-1 daily oral doses of aqueous pods extract of Acacia nilotica affect male fertility in the rabbit model via a severe decrease in the serum testosterone levels.
KEYWORDS: Acacia nilotica, bark, flowers, leaves, pods, serum testosterone, male rabbits
INTRODUCTION
An increase in the world’s population at dramatic rates is a matter of great global concern as the resources are consumed at speed of 1.7 times more than the planet’s capacity, while the demands for the natural resources are increased by three times [1-3]. Family planning programs have been initiated in many countries as per the WHO guidelines to control and to achieve sustainable human reproduction, leading to reinforcement of human rights, economic growth, regional development and environmental protection [4]. The main goal is to avoid unintended pregnancies by following safe and effective contraception. Unfortunately, females have to bear the burden of finance, health and other related issues. The overall cost of female contraceptives is higher than the male contraceptives which include physician visits and prescriptions; moreover, it requires knowledge, time, energy and cares [5,6]. To equalize reproductive autonomy and burden the responsibility should be shared by both partners. The available male contraceptive methods include the pull-out method, condoms and vasectomy. Vasectomy provides irreversible contraception while the pulls out method and use of condoms have higher failure rates [7]. Development of male contraceptive pills such as Nestorone-Testosterone Gel, oral dimethandrolone undecanoate DMAU is currently under clinical trials [8,9]. Reversible Inhibition of Sperm Under Guidance, RISUG® administered by injection is a copolymer of maleic anhydride and styrene. It provides long term contraceptive effects, the product is under phase III clinical trials [10,11]. In contrast, extracts of herbal drugs are being used as folk medicine since ancient times which are still used in areas where modern medicines are not accessible and it is considered to have lesser side effects [12]. Herbal contraceptives are used in Africa and Pacific Asia including China, India, Pakistan and Bangladesh but scientific data on their safety and efficacy are not well established.
According to literature studies Acacia nilotica is considered to have significant male anti-fertility characteristics [13]. It is commonly known as Gum Arabic in Arabic, Babool, Indian gum, and Kikar in Hindi, Urdu and Bengali. The genus Acacia with approximately 1350 species is considered the second largest in the family Leguminosae. It grows in warm and tropical areas of Australia, Africa, Asia and America [14]. Acacia nilotica is native to India, Bangladesh, Pakistan, Nepal and Myanmar and is also grown in Angola Egypt, Ethiopia and Tanzania. It is a perennial tree with a medium average height of 4-7m. The crown of this tree is moderately dense and rounded or flattened. In mature trees, the colour of the bark is dark brown or blackish-green with longitudinal fissures. Acacia nilotica is abundantly found in Sindh, Pakistan. The plant is well-known as a multipurpose tree in several parts of the world which is used for environmental management, in preparation of chemicals, as a source of food, drink, fibre, wood [15]. The parts of the tree are digestible and rich in proteins. The leaves of this tree are used for its antibacterial activity and its ethanolic, methanolic, aqueous, chloroform and petroleum extracts have both antibacterial and antifungal properties, these activities against various pathogens are according to the composition of the extracts. Tender leaves and pods are effective for the treatment of diarrhoea because of anti-dysenteric and antispasmodic activities [16]. Pods of this tree are used as a contraceptive in Africa, due to its antifertility effects on males [17].
Male fertility depends on the release of hormone Testosterone from the Leydig cells of the testes and the initiation of the process of Spermatogenesis process. Testosterone is a steroid hormone and its levels vary with age and time; with an increase in age, its levels decrease. The concentration reaches the highest at the age of puberty. Its concentrations are observed to be higher in the morning [18]. In the process of Spermatids, Synthesis testosterone acts upon the Sertoli cells in the testes to provide metabolic support during the synthesis and it also stimulates the process of sperms maturation [19]. In low levels or complete reduction or a severe increase in the testosterone levels, meiotic cell division, sperm cells development and maturation will not proceed. In comparison to its concentration in the testes, the concentration of testosterone in blood plasma is 25 to 125 times more [20]. In adult males, the secretion of testosterone is 4- 9 mg per day. The total concentration of testosterone in blood plasma is 400-600 ng/dL. 60% binds to plasma albumin and 40% binds to SHBG (sex hormone-binding globulin), while only 2% is freely available to bind to the intracellular receptors. Its levels are decreased in the blood in half to one hour. In the cells it is metabolized into dihydrotestosterone and estradiol; the affinity of dihydrotestosterone is more for androgen receptors in tissues. Testosterone is metabolized in the liver and excreted in urine [21]. A testosterone serum concentration is related to its volume and levels in the testes [22].
The objective of the current study was to evaluate the effects of different parts on serum testosterone levels of male rabbits.
MATERIAL AND METHODS
Study Area
The current study was conducted at the Department of Pharmacology, Faculty of Pharmacy, Hamdard University Karachi from August-November 2019.
Plant collection
Bark, leaves, flowers and pods of Acacia nilotica were purchased from a local vendor and were identified by the Botanical Garden of Hamdard University, Karachi. The collected parts were washed with distilled water, dried under shade and then ground separately using an electric grinder to obtain a homogenous powder and weighed. Obtained powder from each crude part was stored in separate labelled glass jars.
Extract preparation
Aqueous extracts of crude bark, leaves, flowers and pods were prepared by maceration [23]100g of dried powder of bark, leaves, flowers and pods were poured separately in 500 ml of distilled water. The suspension was stirred, covered and kept for three days at room temperature.The prepared suspensions were decanted to separate sediments and impurities and then filtered using muslin cloths. All obtained solutions were dried in a water bath at 50°C to obtain crystals.
To prepare alcoholic extracts of Acacia nilotica bark, leaves, flowers and pods extract maceration method of extraction was used [24]. 100 g of dry powder of bark, leaves, flowers and pods were soaked in 500 ml of 75% separately. After stirring the suspensions were covered with aluminium foils. The suspensions were kept at room temperature for up to three days. After three days the suspensions were decanted and separately filtered through muslin cloths. The impurities and sediments were discarded. To obtain solid crystals the resultant solutions were evaporated at room temperature. The yields of the prepared aqueous and ethanol extracts were calculated by the formula, [23]
Percentage Yield = (Weight of prepared extract in grams / 100 grams of dry crude powder) x 100
The prepared extracts were collected in a separate labelled glass jar, closed with lids. They were stored in the refrigerator at 2 - 4° C.
Study animals
We have selected 45 healthy adult male rabbits (Oryctolagus cuniculus) age between 1- 1.5 years for this experiment. All selected animals were housed in separate cages tagged with their assigned numbers. 12 hours of light and dark cycles were maintained with a temperature range of 20 – 22 degrees Celsius. Rabbits were fed with fresh vegetables, hay and freshwater ad libitum.
Treatment groups
The study was approved by the Ethical Review Board of Hamdard University. Selected animals were divided into nine groups, having five rabbits in each group. Rabbits in all mentioned designated treatment groups received the following treatments for up to eight weeks period.
- Group, I(b) received oral daily doses of 400 mg of prepared aqueous extract of Acacia nilotica bark per kilograms weight of the rabbit.
- Group I(f) received oral daily doses of 400 mg of prepared aqueous extract of Acacia nilotica flowers per kilograms weight of the rabbit.
- Group I(L) received oral daily doses of 400 mg of prepared aqueous extract of Acacia nilotica leaves per kilograms weight of the rabbit.
- Group, I(p) received oral daily doses of 400 mg of prepared aqueous extract of Acacia nilotica pods per kilograms weight of the rabbit.
- Group II(b) received oral daily doses of 400 mg of Acacia nilotica bark extract prepared in 75 % ethanol, per kilograms weight of the rabbit.
- Group II(f) received oral daily doses of 400 mg of Acacia nilotica flower extract prepared in 75 % ethanol, per kilograms weight of the rabbit.
- Group II(L) received oral daily doses of 400 mg of Acacia nilotica leaves extract prepared in 75 % ethanol, per kilograms weight of the rabbit.
- Group II(p) received oral daily doses of 400 mg of Acacia nilotica pods extract prepared in 75 % ethanol, per kilograms weight of the rabbit.
- Control Group III received oral 3 ml of Normal Saline daily.
Blood collection
Blood samples were collected according to standard procedures [25], from all the rabbits for the baseline; second, fourth, sixth and eighth week of treatment. For blood collection the rabbits were put in the restrainer, their ear was cleaned with spirit and Lignocaine Gel was applied. After 10 minutes, 2 mL of blood was drawn through a 26 Gauge butterfly needle (Huamei, China) from the of the ear into the labelled collection tubes. To stop the bleeding a piece of sterile cotton wool was applied with figure pressure.
Serum hormonal assay
Blood samples were assayed at the testing laboratory facility at Taj Medical Complex, Hamdard University Hospital located at Muhammad Ali Jinnah Road, Karachi. ELISA (Enzyme-Linked Immunosorbent Assay) technique was used for the quantitative analysis of serum testosterone concentrations [26,27]. Blood samples were centrifuged and stored at -80°C. DiaMetra® Testosterone ELISA kits, Italy was used for serum testosterone assay; it has an analytical range of 0.2 – 16 ng / mL. The sensitivity of the serum hormonal assay was 0.07 ng / mL according to the user manual guide. Pooled serum (serum of 5 rabbits were mixed in the Control Group) was tested to validate the procedure of assay.
Statistical analysis
All the data were presented as Mean +SD by SPSS -21. One way ANOVA followed by post hoc Tukey HSD. P <0.05 was considered significant.
RESULTS AND DISCUSSION
GROUP I (b)
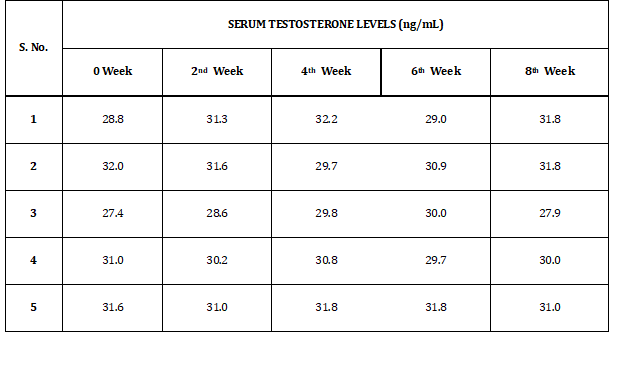
Table 1 shows the results of serum testosterone levels of rabbits at baseline, second, fourth, sixth and eighth week. Levels of serum testosterone levels vary from 28.8 to 31.8 ng/dl.
Table 1: Serum Testosterone Levels of Group I (b)
GROUP I (f)
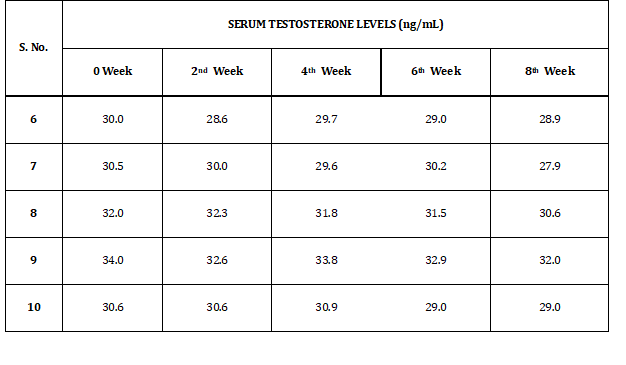
Table 2 shows the test results of serum testosterone levels of rabbits obtained during eight weeks of study. Levels of serum testosterone levels vary from 27.9 to 34 ng/dl.
Table 2: Serum Testosterone Levels of Group I (f)
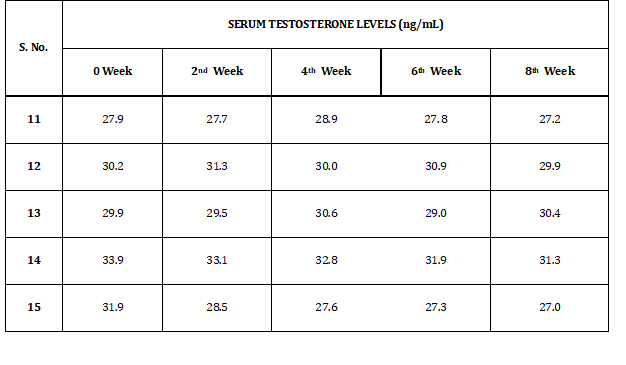
Table 3 indicates serum testosterone levels of rabbits in group I (L). Levels of serum testosterone levels vary from 27 to 33.9 ng/dl.GROUP I (L)
Table 3: Serum Testosterone Levels of Group I (L)
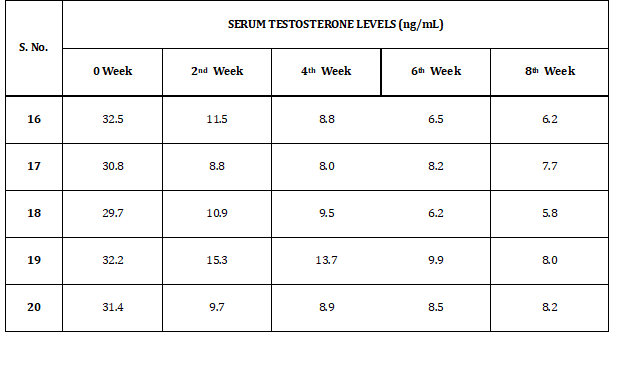
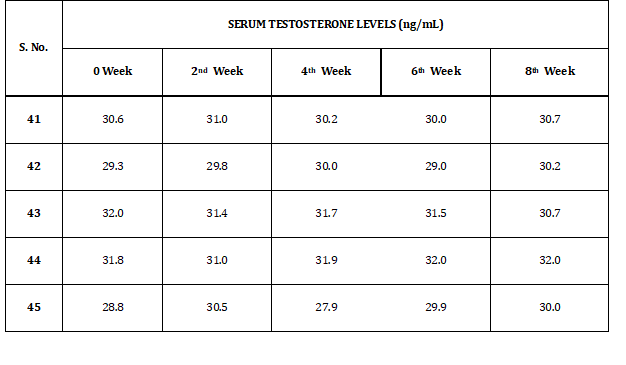
GROUP I (p)
Table 4 represents the results of serum testosterone levels of rabbits in group I (p). Levels of serum testosterone levels vary from 5.8 to 32.5 ng/dl.
Table 4: Serum Testosterone Levels of Group I (p)
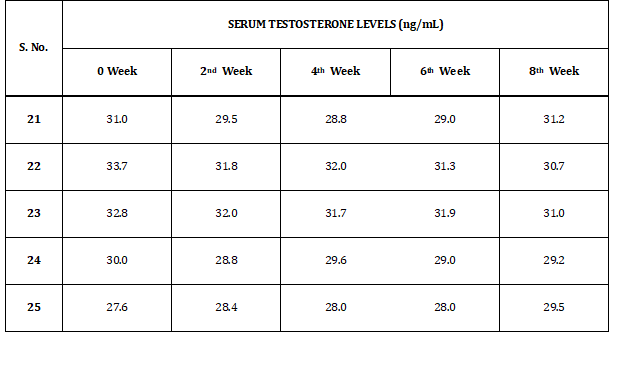
Table 5 shows the test results of serum testosterone levels of rabbits in group II (b). Levels of serum testosterone levels vary from 27.6 to 33.7 ng/dl.GROUP II (b)
Table 5: Serum Testosterone Levels of Group II (b)
Table 6 refers to the test results of serum testosterone levels of rabbits included in group II (f). Levels of serum testosterone levels vary from 27.6 to 32 ng/dl.GROUP II (f)
Table 6: Serum Testosterone Levels of Group II (f)
Table 7 indicates serum testosterone levels of rabbits in group II (L). Levels of serum testosterone levels vary from 28.5 to 32.4 ng/dl.GROUP II (L)
Table 7: Serum Testosterone Levels of Group II (L)
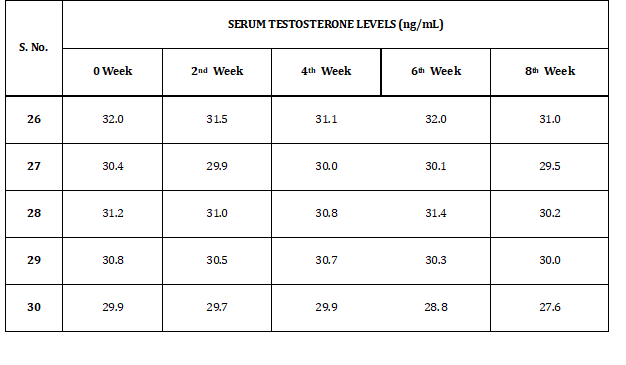
Table 8 refers to the obtained serum testosterone levels of the rabbits in group II (p). Levels of serum testosterone levels vary from 28.5 to 32.9 ng/dl.GROUP II (p)
Table 8: Serum Testosterone Levels of Group II (p)
Refer to Table 9 for the serum testosterone levels of the rabbits of the control group III. Levels of serum testosterone levels vary from 28.8 to 32.0 ng/dl.CONTROL GROUP III
Table 9: Serum Testosterone Levels of Control Group III
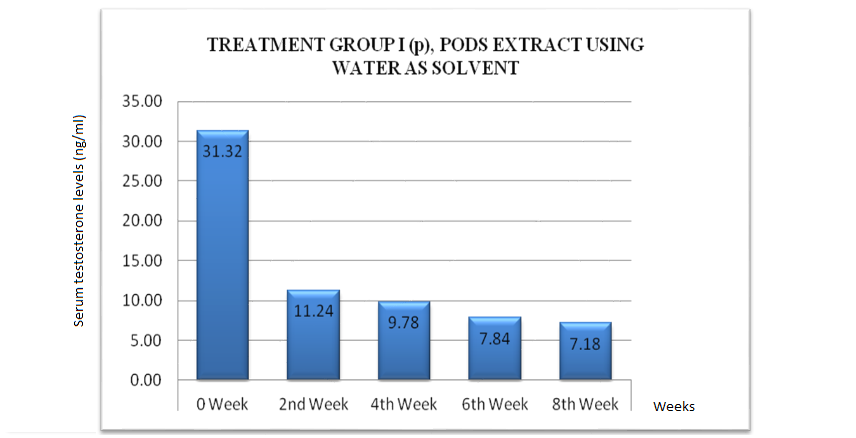
Testosterone plays a vital role in male fertility. It helps in the synthesis of spermatids, in the maturation of sperms and in the development of male characteristics in young males. The results demonstrated that a significant decrease in serum testosterone concentrations was observed in the rabbits that had been administered aqueous extract of Acacia nilotica pods in oral daily doses of 400 mg kg-1 till eight weeks. A decrease in 64.1 % of serum testosterone levels was noticed in the second week of its administration when compared with the Control Group III, followed by a further decrease by 68.7 % on the fourth week, 74.9 % on the sixth week and 77.0 % on the eighth week of the treatment. The pattern of decrease from the baseline till the eighth week of the treatment was represented Fig. 1, after oral administration of a daily dose of aqueous extract of Acacia nilotica pods in the adult mature male rabbits of Group I (p).
Figure 1: Decrease in serum testosterone levels in treatment group I (p)
The test results of Group I(p) were statistically significant with a p-value < 0.05, in a similar study significant decrease in serum testosterone levels, were achieved in adult male Wistar rats who had received Acacia nilotica extract, published by Lampiao, et al.[28] and Kadhem, et al.[29], concluded that the extract of Anethum graveolens, Dill, has shown antifertility effects on adult male rabbits. In a similar study Njoku, et al.[30] published in 2021, it is reported that extract of Talpa occidentalis, fluted pumpkin seeds and leaves significantly decreased serum testosterone levels in male mice at higher doses.
The presence of carbohydrates, alkaloids, flavonoids, sterols, saponins and tannins in the aqueous extract of Acacia nilotica pods [31-33] needs further analysis and identification of the active constituent which mimics the male antifertility effecn ts and is water-soluble.
In a previous study, Acacia nilotica treatment for 16 weeks result in decreased total sperm motility. Moreover normal sperm morphology was also affected with reduction in testosterone level [28]. Another study reported that up to 40% Acacia leaves can be used effectively in the diet of rabbits without significant effect on sexual activity of animals [34]. Simailarly another study revealed that Acacia hydaspica treatment in Cisplatin induced testicular toxicity reverted the level of fertility hormones [35].
Serum Testosterone concentrations in the rabbits of Group I(f) demonstrated a slight insignificant decrease at the eighth week (p-value > 0.05). They have been administered oral daily 400 mg/ kg doses of aqueous flower extract of Acacia nilotica for up to eight weeks. Insignificant changes in the serum testosterone levels were observed in Groups II (b), II (f), II (l) and II (p) rabbits that received extracts of Acacia nilotica bark, flowers, leaves and pods prepared using 75 % ethanol as solvent (p-value > 0.05).
The current study was conducted to evaluate the effect of different extracts of different parts of Acacia nilotica plant on serum testosterone levels of male rabbits. As significant effects were observed, it carn be used for different male sexual problems. Further it can be utilised to develope male contraceptive in future. We recommend to conduct further studies in future on large scale along with mechanism exploration to get significant benefit from this plant in male sexual diseases.
CONCLUSION
Aqueous extract Acacia nilotica pods in 400 mg/kg oral daily doses up to eight weeks severely decreased serum testosterone concentrations in adult male rabbits, making it a potential herb with male antifertility effects. Further studies are required to identify the active component, its mechanism of action, safety, efficacy as well as reversibility.
SIGNIFICANCE STATEMENT
This study discovers the effects of different parts of the Acacia nilotica plant on the serum testosterone levels of male rabbits that can be beneficial for male sexual problems. Thus a new theory on the role of this plant on male hormone i.e. testosterone is arrived at.
AUTHOR’S CONTRIBUTION
Syed Muhammad Imran Shah: carried out research, compiled the data and wrote manuscript; Ishrat Younus: designed and supervised the study; Laila Anwer: co-supervised the research project; Faiza Fakhar: Helped in data analysi; Farzana Sadaf: helped in animal handling and review the manuscript.
CONFLICT OF INTEREST
Authors declared that they have no conflict of interest.
REFERENCES
-
Augustine BC, Kéry M, Olano Marin J, Mollet P, Pasinelli G, et al. (2020). Sex‐specific population dynamics and demography of capercaillie (Tetrao urogallus L.) in a patchy environment. Population Ecology. 62(1):80-90. [Link]
-
Grow A & van Bavel TJ (Eds.). (2017). Agent-Based Modelling in Population Studies. Springer. [Link]
-
Nadakavukaren A and Caravanos J. (2020). Our global environment: A health perspective. Waveland Press.
-
Cleland J, Bernstein S, Ezeh A, Faundes A, Glasier A, et al. (2006). Family planning: the unfinished agenda. Lancet 368(9549):1810-1827.
-
Amory JK. (2016). Male contraception. Fertility and sterility. 106(6):1303-1309.
-
Zaman W, Ahmad M, Zafar M, Amina H, Ullah F, et al. (2020). The quest for some novel antifertility herbals used as male contraceptives in district Shangla, Pakistan. Acta Ecologica Sinica. 40(1):102-112.
-
RP Tulsiani D and Abou-Haila A. (2014). Importance of male fertility control in family planning. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 14(2):134-144.
-
Thirumalai A, Ceponis J, Amory JK, Swerdloff R, Surampudi V, et al. (2019). Effects of 28 days of oral Dimethandrolone Undecanoate in healthy men: a prototype male pill. The Journal of Clinical Endocrinology & Metabolism. 104(2):423-432.
-
Khilwani B, Badar A, Ansari AS, and Lohiya NK. (2020). RISUG® as a male contraceptive: journey from bench to bedside. Basic and Clinical Andrology. 30(1):1-12.
-
Sharma RS, Mathur AK, Singh R, Das HC, Singh GJ, et al. (2019). Safety & efficacy of an intravasal, one-time injectable & non-hormonal male contraceptive (RISUG): A clinical experience. The Indian Journal of Medical Research. 150(1):81.
-
Ansari AS, Badar A, Balasubramanian K, and Lohiya NK. (2017). Contraception with RISUG® and functional reversal through DMSO and NaHCO3 in male rabbits. Asian journal of andrology. 19(4):389.
-
Solakhia R, Gupta J, and Parashar B. (2019). Herbal Contraceptives: Anti-fertility Activity of Herbal Plants. International Journal of Health and Clinical Research. 2(4):5-10.
-
Plana O. (2017). Male contraception: research, new methods, and implications for marginalized populations. American journal of men's health. 11(4):1182-1189.
-
Rather LJ, Akhter S, Padder RA, Hassan QP, Hussain M, et al. (2017). Colorful and semi durable antioxidant finish of woolen yarn with tannin rich extract of Acacia nilotica natural dye. Dyes and Pigments. 139:812-819.
-
Bhushette PR and Annapure US. (2017). Comparative study of Acacia nilotica exudate gum and acacia gum. International journal of biological macromolecules. 102:266-271.
-
Zabré G, Kaboré A, Bayala B, Katiki LM, Costa-Júnior LM, et al. (2017). Comparison of the in vitro anthelmintic effects of Acacia nilotica and Acacia raddiana. Parasite. 24:44
-
Deshmukh SP, Shrivastava B, and Bhajipale NS. (2018). A Review on Acacia species of therapeutics importance. International Journal of Pharmaceutical and Biological Science Archive. 6(4):24-34.
-
Baker HWG, Burger HG, De Kretser DM, Hudson B, O'connor S, et al. (1976). Changes in the pituitary‐testicular system with age. Clinical endocrinology. 5(4):349-372. [Link]
-
Katragadda V, Adem M, Mohammad RA, Bhasyam SS, and Battini K. (2021). Testosterone recuperates deteriorated male fertility in cypermethrin intoxicated rats. Toxicological Research. 37(1):125-134. [Link]
-
Walker WH. (2011). Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 1(2):116-120.
-
Brunton L, Chabner B, and Knollmann B. (2011). Goodman & Gilman's the pharmacological basis of therapeutics, 12th ed., McGraw Hill Companies, pp.1195-1199.
-
Ruiz‐Olvera SF, Rajmil O, Sanchez‐Curbelo JR, Vinay J, Rodriguez‐Espinosa J, et al. (2018). Association of serum testosterone levels and testicular volume in adult patients. Andrologia. 50(3):12933.
-
Pandey A and Tripathi S. (2014). Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry. 2(5):115-119.
-
Mohammed HA. (2020). Behavioral Evaluation of the Effects of Aqueous and Ethanol Extracts of Suaeda vermiculata Forssk on Rats. Central Nervous System Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Central Nervous System Agents). 20(2):122-127.
-
Parasuraman S, Raveendran R and Kesavan R. (2010). Blood sample collection in small laboratory animals. Journal of pharmacology & pharmacotherapeutics. 1(2):87.
-
Arteaga L, Bautista A, Martínez-Gómez M, Nicolás L, and Hudson R. (2008). Scent marking, dominance and serum testosterone levels in male domestic rabbits. Physiology & behavior. 94(3):510-515.
-
Shrivastav TG, Basu A, and Kariya KP. (2003). One step enzyme linked immunosorbent assay for direct estimation of serum testosterone. Journal of Immunoassay and Immunochemistry. 24(2):205-217.
-
Lampiao F. (2013). The anti-fertility effects of Acacia nilotica in male Wistar rats. Journal of reproduction & infertility. 14(1):39.
-
Kadhem MA, Alrekabi AA, and Khassaf HK. (2020). Anti-fertility Effects of Ethanolic Extract of Anethumgraveolens in Male Rabbits. Medico Legal Update. 20(4):1927-1932.
-
Njoku RCC and Abarikwu SO. (2021). Antifertility and profertility effects of the leaves and seeds of fluted pumpkin: Sperm quality, hormonal effects and histomorphological changes in the testes of experimental animal models. Journal of Integrative Medicine. 19 (2):104-110
-
Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, et al. (2014). Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). In Veterinary research forum: an international quarterly journal. 5(2):95.
-
Sadiq MB, Hanpithakpong W, Tarning J and Anal AK. (2015). Screening of phytochemicals and in vitro evaluation of antibacterial and antioxidant activities of leaves, pods and bark extracts of Acacia nilotica (L.) Del. Industrial Crops and Products. 77:873-882.
-
Jame R. (2018). Phytochemical and Pharmacological Uses of Acacia Nilotica - A Review. International Journal of Bioorganic Chemistry. 3(2):6-10.
-
Yousef MI. (2005). Reproductive performance, blood testosterone, lipid peroxidation and seminal plasma biochemistry of rabbits as affected by feeding Acacia saligna under subtropical conditions. Food and chemical toxicology. 43(2):333-339.
-
Afsar T, Razak S, and Almajwal A. (2017). Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC cancer. 17(1):1-14.
Copyright: Younus I, et al. © (2021).. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Younus I, et al. (2021). Effect of Different Parts of Acacia Nilotica on Serum Testosterone Levels in Male Rabbits Pharmacogn. 1(1):2.
 Abstract
Abstract  PDF
PDF