Past Issues
Formulation Design and Optimization of Poly-herbal Chewable Tablets Using JMP Tool
Pramod J Hurkadale*, Nutan M. Channi, Udaykumar B. Bolmal and Chitrali M. Bidikar
Department of Pharmacognosy, KLE College of Pharmacy , KLE Academy of Higher Education and Research Nehru Nagar , Belagavi - 590010, Karnataka, India
*Corresponding Author: Dr. Pramod HJ, Professor, Department of Pharmacognosy, KLE College of Pharmacy, KLE Academy of Higher Education and Research, Nehru Nagar, Belagavi - 590010, Karnataka, India; Tel: 9448244252; Email: [email protected]
Received Date: May 17, 2023
Publication Date: October 21, 2023
Citation: Channi NM, et al. (2023). Formulation Design and Optimization of Poly-Herbal Chewable Tablet Using Jmp Tool. Pharmacogn. 2(1):3.
Copyright: Channi NM, et al. © (2023).
ABSTRACT
Background and Objectives: Usage of traditional medicines increases when conventional medicines are ineffective. The present study was aimed to develop a formulation design and optimization of poly-herbal chewable tablet using different medicinal plant extracts against infectious disease. Methods: A total of eight medicinal plants were selected and named Myrica nagi (M. nagi), Clerodendrum serratum (C. serratum), Inula racemose (I. racemose), Piper nigrum (P. nigrum), Terminalia bellerica (T. bellerica), Pistacia integerrima (P. integerrima), Solanum indicum (S. indicum), Acacia catechu (A. catechu). These plants are extracted separately. We designed the development of poly-herbal chewable tablets using JMP software and investigated the antimicrobial activity of the optimized poly-herbal chewable tablet. Results: The antimicrobial activity of the optimized poly-herbal chewable tablet was investigated and the formulation development of the poly-herbal chewable tablets was designed using the JMP Trial 16.0 software. The pre-formulation and post-formulation parameters studied show that the CT 07, CT 09, and CT 11 formulations show best results from all the formulations were designed. Stability study results that optimized poly-herbal chewable tablet were stable throughout the study. Interpretation & Conclusion: the outcome of the formulation show that CT 07, CT 09 and CT 11 are the better formulations, all the extracts have antimicrobial activity against infectious disease and have a positive effect on the treatment of upper respiratory tract infection, based upon the tablet evaluation parameters.
Keywords: Anti-microbial activity, Chewable tablet, Polyherbal, JMP Software
INTRODUCTION
The current trend towards long term healthy living relies entirely on the traditional health system. The Indian philosophy behind Ayurveda is to prevent unnecessary survival pains by curing human disease, also known as an important centre of biodiversity through around 45,000 herbal remedies, of which around 15,000 herbal plants use a single plant, which has been recorded. The effective phytochemicals of individual plants are not sufficient to achieve beneficial effects; therefore the combination of several herbs (polyherbal) in specific proportions will give the ideal therapeutic effect. Multi-herbs preparation contains two or more herbs with different botanical compositions these herbs have similar or different therapeutic potential and together provide ideal effects in the treatment of human diseases. Herbal preparations made from plants are effective in low doses and safe in high doses due to the wide range of treatments available. Improper use has fewer side effects, but it is extremely popular [1].
Generally soreness is represented by the patient swallow the pain in the throat without any effort as well as pain occurs while swallowing. Bacterial sore throats caused by the GAS (Group A beta – haemolytic Streptococcus) it reports that acute Pharyngitis shows 12-36% in children and in India 13.4% of cases are reported [2].
Chewable tablets should be ground and chewed between teeth ingest. Chewable tablets are solid formulations which generally contain one or more sweetening and flavouring agents and are designed to slowly dissolve or eliminate the characteristic odour of the oral cavity. Chewable tablets can be prepared by molding or compressing sugar-based materials. Most chewable tablets are available OTC (over the counter). Chewable tablets provide a comfortable dosage form administration method and benefit from their position in the pharmaceutical market due to their various advantages [3].
The chewable tablets are more stable and when they start to disintegrate in the oral cavity and slowly release the drug, in this case the active ingredients of the drug is seen and affects the bacterial present. 13 different chewable tablet formulations were prepared by varying the composition of the tablets and quality control testing was performed on the formulations including friability, hardness, disintegration time, and changes in weight. The antibacterial activity of the preparation against streptococcus was tested [4].
MATERIALS & METHODS
Materials
Kathphala (M. nagi), Bharangi (C. serratum), Pushkarmoola (I. racemosa), Maricha (P. nigrum), Bibhikati (T. bellerica), Kakarsinghi (P. integerrima), Brihati (S. indicum), Khadira (A. catechu) were collected from the local areas of the Belagavi (India) and authenticated in Shri B.M.K. Ayurveda Mahavidyalaya. Belagavi.
Method
Collection and Extraction
All raw materials/crude drugs were dried for 14 days at room temperature. Made a coarse powder out from the dried raw materials. To soak the coarse powder, added 70% ethanol. After a 48 hrs of cold maceration, the extract was filtered. The marc was used for subsequent extraction with a Soxhlet extractor using 95% ethanol. Combined the filtrate collected by cold maceration and soxhlet extract. Then under pressure, used a rotary evaporator (IKA RV 10) at 40℃ to concentrate the combined filtrate. The concentrated extract was then retrieved from the rotary evaporator and placed in a water bath to evaporate any leftover solvent.
Phyto-chemical Analysis [5]
All crude drugs components were subjected to phyto-chemical analysis in order to identify classes
Physico-chemical evaluation
All crude drugs were subjected to Physico-chemical evaluation such as,
Total Ash Value, Acid Insoluble Ash Value, Water Soluble Ash Value, Water-Soluble Extractive Values,
Alcohol-Soluble Extractive Values, LOD (Loss on Drying)
Pre-compression parameter [6]
Angle of repose
The funnel method is used to determine the powders angle of repose. Pour the powdered ingredients into a funnel and thoroughly mix. Adjust the funnel so that the cone stacks tip contacts the funnels bottom. Measure the diameter and height of the mixed piles bottom and repeat the process three times to get the average diameter. In Addition, the angle of repose must be calculated with help of formula.
Tan θ = height of the powder cone / radius of the powder cone
LBD (Loose bulk density)
Filling a graduated cylinder with a known amount of powder yields the bulk density of the powder. Calculate the density using the formula after measuring the volume of the graduated cylinder.
LBD = Mass of powder / volume of the powder in the graduated cylinder
TBD (Tapped bulk density)
A graduated cylinder holding particles of known mass is used to determine this. At 2 second intervals, drop the cylinder from a height of 10cm onto ahard surface under its own weight. Continue tapping until there is no more volume change.
TBD = Quantity of powder withdrawn / final quantity of powder after tapping
Carr’s compressibility index
Replace the results obtained from the bulk density and the tapped density in the equation to calculate the compressibility.
Carr’s compressibility index = 100×(A1 – A2)/ A1
Where A1 = initial volume of the powder before tapping process, and A2 = Final volume of the powder after tapping process
Hausner ratio
The hausner ratio is the ratio of starting powder volume to the end powder volume following the tapping process.
Hausner ratio = A0 / Af
Where, A0 = initial volume of the powder before tapping process, and Af = Final volume of the powder after tapping process
JMP Software [7]
This custom design applied to formulation design for poly-herbal chewable tablets. Two different factors are evaluated in this design. The two independent variables selected were T. bellerica as the binder and P. nigrum as the disintegrate. The possible dependent variables are Hardness, Friability, and Disintegration time of the designed and formulated poly-herbal chewable tablets. For theformulation design, JMP trial version 16 software (SAS Institute Inc, Seattle, US) was used to design a total of 13 analyzes (formulations), the relationship between the dependent variable and independent variable was investigated, a surface response was obtained and a meaningful model was finally established. Formulation table shown in Table 1.
Table 1: Formulation Table.
|
Ingredients |
CT 01 mg |
CT 02 mg |
CT 03 mg |
CT 04 mg |
CT 05 mg |
CT 06 mg |
CT 07 mg |
CT 08 mg |
CT 09 mg |
CT 10 mg |
CT 11 mg |
CT 12 mg |
CT 13 mg |
|
Myrica nagi |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Clerodendrum Serratum |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Inula racemosa |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Terminalia bellerica |
10 |
2 |
2 |
10 |
2 |
2 |
6 |
10 |
6 |
6 |
6 |
6 |
10 |
|
Pistacia integerrima |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Piper nigrum |
3 |
2 |
3 |
1 |
3 |
1 |
2 |
1 |
2 |
3 |
2 |
1 |
2.15
|
|
Solanum indicum |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
Acacia catechu |
34 |
34 |
34 |
34 |
34 |
34 |
34 |
34 |
34 |
34 |
34 |
34 |
34 |
|
Stevia |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
|
Mentha piperita L |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
4 |
|
Carboxyl methyl cellulose |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
q/s |
|
Total |
100
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
100
|
Independent levels: T. bellerica (X1)
P. nigrum (X2)
Dependent levels: Hardness (Y1)
Friability (Y2)
Post-compression parameters [8]
The average of all of the post-compression study carried out in triplicate the ultimate outcome was determined.
Hardness
The hardness of several tablets was determined use a Monsanto. The tablet hardness is measured in kg/cm2
Thickness
Measured the thickness of the tablets with a vernier calliper and recorded it in mm.
Diameter
Measured the Hardness of the tablet with a vernier calliper and recorded it in mm.
Friability
Tablet friability research is conducted using the Roche friability tablet machine. Took 20 tablets from each batch, weighed them, and recorded their beginning weight. The tablet was then loaded into the device, which was then loaded into the device, which was then run for 4 minute or 100 revolutions (25rpm). Finally, remove the tablet, weight it and record the final weight, then calculate the friability using the following formula:
% of friability = (initial weight – final weight) / initial weight × 100
Weight variation
To evaluate the weight change in the tablet formulation, 20 tablets from each batch were individually weighed and the weight reported. The average tablet weight was determined and then entered into the equation. The average tablet weight was computed and then substituted in to the formula.
Wight change = (weight of single tablet / average weight of the tablet) × 100
Disintegration
Using a disintegrator, disintegrate the tablets, fill the disintegration vessel with 900ml of distilled water, and remove 6 tablets from each batch and load them into the device. The temperature is kept constant at 37±2℃, the frequency is set to 28-32 cycles / minutes, and the tablet disintegration time is limited to 15 minutes.
Accelerated stability test
The stability of the prepared poly-herbal chewable tablets is evaluated on the 7th, 15th, and 30th days at 25℃±2℃ / RH 60±5 %( room temperature) and 40℃±2℃ / RH 75±5 %( accelerated temperature).
Anti-microbial activity [9]
The agar well diffusion method is used to accomplish this. With a non-toxic cotton swab, spread the fresh microbiological culture (Staphylococcus aureus) on a Muller Hinton agar plate. Make 10 holes 6mm in diameter on the agar medium with a sterile cork drill (6mm) and fill each hole with 1000µg/ml of plant extracts using micropipette under sterile circumstances. Ciprofloxacin (5µl) was utilised as the standard medication, with ethanol serving as a negative control. Pre-diffuse the extracts in the medium for 1 hour on the plates. Antimicrobial detection was determined by incubating the plate vertically for 24 to 48 hours at 37℃±2℃ aerobic and measuring the inhibition zone (mm).
RESULTS AND DISCUSSION
Phyto-Chemical Investigation
The phyto-chemical investigations are the first step in the pre-formulation studies. The 8 extracts each have their own qualitative screening of phyto-chemicals. Alkaloids, Carbohydrates, Flavonoids, Tannins, and Steroids are among the principal classes screened qualitatively. The extract of M. nagi contained Alkaloids; the extract of C. serratum contained Alkaloids, Flavonoids, and Tannins; the extract of I. racemosa contained Alkaloids, Carbohydrates, Flavanoids, Tannins, and Steroids; the extract of P. nigrum contained Alkaloids, Flavonoids, Tannins, and Steroids; the extract of T.bellerica contained Alkaloids, Flavonoids, Tannins; the extract of P. intergerrima had Carbohydrates, Flavonoids, Tannins; the extract of S. indicum had Alkaloids, Steroids; and the extract of A. catechu contained Alkaloids, Carbohydrates, Flavonoids, Tannins, and Steroids. Phyto-chemical investigation results are shown in Table 2.
Table 2: Phyto-chemical investigation results.
|
Tests |
Alkaloids |
Carbohydrates |
Flavonoids |
Tannins |
Steroids |
|
Myrica nagi |
+ |
- |
- |
- |
- |
|
Clerodendrum serratum |
+ |
- |
+ |
+ |
- |
|
Innula racemosa |
+ |
+ |
+ |
+ |
+ |
|
Piper nigrum |
+ |
- |
+ |
+ |
+ |
|
Terminalia bellerica |
+ |
- |
+ |
+ |
- |
|
Pistacia intergerrima |
- |
+ |
+ |
+ |
- |
|
Solanum indicum |
+ |
- |
- |
- |
+ |
|
Acacia catechu |
+ |
+ |
+ |
+ |
+ |
Physico-Chemical Investigation
One of the most important investigation tests in the pre-formulation field of herbs and extracts is the physico-chemical research. LOD, Ash value (Total ash, Water soluble ash value, and Acid soluble ash value). Alcohol soluble extractive value and water soluble extractive values were the parameter investigated in the physic-chemical studies. For the physic-chemical investigations, the extracts are handles individually. Table 3 summarises the findings. I. racemosa (6.66%) had the highest % of Loss on drying (LOD), followed by M. nagi (4%), C. serratum (4.66), P. nigrum (1.33%), T. bellerica (4%), P. integerrima (2%), S. indicum (4%), and A. catechu (0.66%). In case of total ash value results were, M. nagi and P. integerrima (3%), I. racemosa, T. bellerica and S. indicum (4.5%), P. nigrum (4%), A. catechu (2.5%) and C. serratum had the highest total Ash value. In the case of the Acid insoluble Ash value results were C. serratum (1%), S. indicum (1.5%) M. nagi, I. racemosa, P. nigrum, T. bellerica, P. integerrima and A. catechu showed up with the same percentage value of 0.5%, whereas in the case of water soluble ash value C. serratum (3.5%), M. nagi and P. integerrima (1%), I. racemosa and A. catechu (2%), P. nigrum (3%), T. bellerica (1.5%) and S. indicum (0.5%). Alcohol soluble extract and water-soluble extract were used to determine the Extractive value parameters, in the case of alcohol soluble extractive values M. nagi (25%), C. serratum (18%), P. nigrum (16%), T. bellerica (39%), P. integerrima (98%), S. indicum and A. catechu (12%), I. racemosa had the lowest Alcohol soluble extractive value (11%). Whereas A. catechu (4%), had the moderate water soluble extractive value followed by M. nagi and C. serratum (17%), I. racemosa (34%), P. nigrum (18%), T. bellerica (53%), P. integerrima (63%), and S. indicum (13%).
Table 3: Physico-chemical investigation results.
|
Parameters |
LOD |
Total ash value |
Acid-insoluble ash value |
Water-soluble ash value |
Alcohol-extractive value |
Water-extractive value |
|
|
Myrica nagi |
4% |
3% |
0.50% |
1% |
25% |
17% |
|
|
Clerodendrum serratum |
4.66% |
5% |
1% |
3.50% |
18% |
17% |
|
|
Inula racemosa |
6.66% |
4.50% |
0.50% |
2% |
11% |
34% |
|
|
Piper nigrum |
1.33% |
4% |
0.50% |
3% |
16% |
18% |
|
|
Terminalia bellerica |
4% |
4.50% |
0.50% |
1.50% |
39% |
53% |
|
|
Pistacia integerrima |
2% |
3% |
0.50% |
1% |
68% |
63% |
|
|
Solanum indicum |
4% |
4.50% |
1.50% |
0.50% |
12% |
13% |
|
|
Acacia catechu |
0.66% |
2.50% |
0.50% |
2% |
12% |
4% |
Pre-compression Parameters
Angle of repose (θ), Loose bulk density (kg/cm3), Tapped bulk density (kg/cm3), Carr’s compressibility index and Hausner ratio were used as pre-compression parameters in the formulation of the polyherbal chewable tablets. It is critical that the pre-compression parameters have a better responsiveness in order for the tablet to compress better. The flow qualities, moisture content and compression parameters are revealed in the pre-compression study. Table 4 shows the data for the pre-compression setting.
Table 4: Pre-compression parameters results.
|
Formulation |
Angle of repose (0) |
Loose bulk density (gm/cm2) |
Tapped bulk density (gm/cm2) |
Carr’s compressibility index |
Hausenr ratio |
|
CT 01 |
0.606 |
||||
|
CT 02 |
|||||
|
CT 03 |
|||||
|
CT 04 |
|||||
|
CT 05 |
|||||
|
CT 06 |
|||||
|
CT 07 |
|||||
|
CT 08 |
|||||
|
CT 09 |
|||||
|
CT 10 |
|||||
|
CT 11 |
|||||
|
CT 12 |
|||||
|
CT 13 |
Post-compression Parameters
The quality control aspect of a compressed tablet is the post compression parameters. The response of the hardness, friability, and disintegrations varies depending up on the binder and disintegrant ratio. Thickness, diameter, and weight fluctuation are the additional quality control metrics given. Table 5 shows the overall outcomes of post-compression.
Table 5: Post-compression parameters results.
|
Formulation |
Hardness (gm/cm2) |
Thickness (mm) |
Diameter (mm) |
Friability (%) |
Weight variation (%) |
Disintegration (min) |
|
CT 01 |
||||||
|
CT 02 |
2.71 |
|||||
|
CT 03 |
||||||
|
CT 04 |
||||||
|
CT 05 |
||||||
|
CT 06 |
||||||
|
CT 07 |
||||||
|
CT 08 |
||||||
|
CT 09 |
||||||
|
CT 10 |
||||||
|
CT 11 |
||||||
|
CT 12 |
||||||
|
CT 13 |
JMP Software
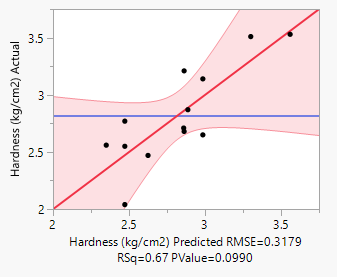
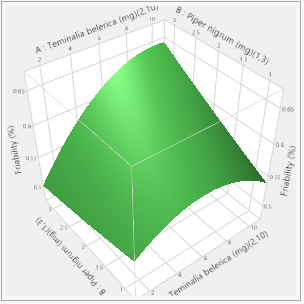
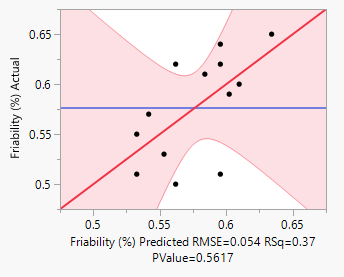
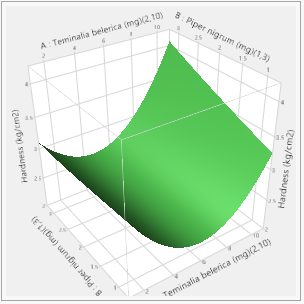
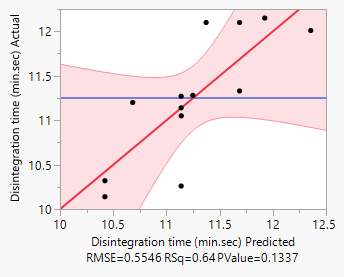
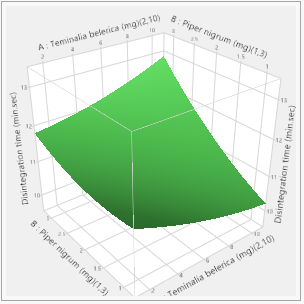
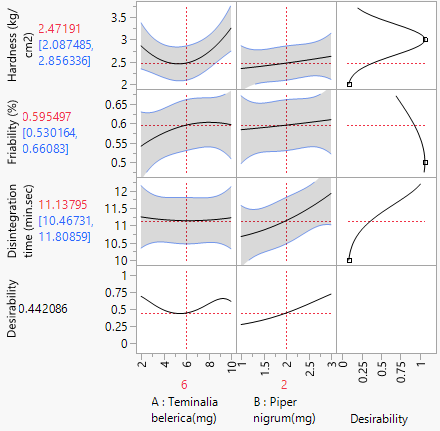
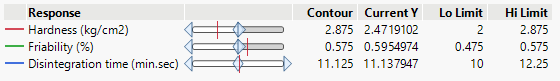
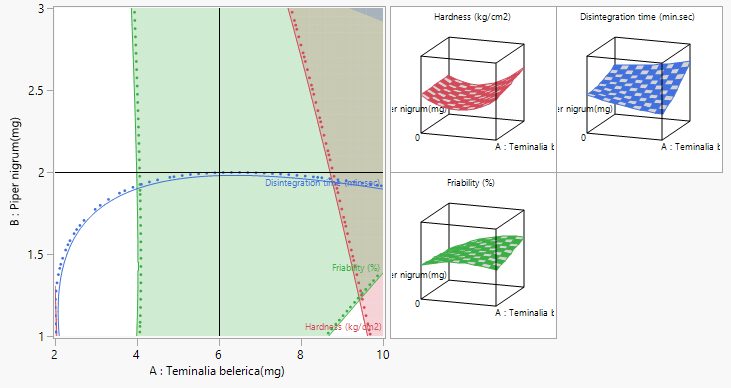
In this study, the formulation design is done with the JMP 16.0 software. The custom design is used, and two independent variables, the binder (T. bellerica) and the disintegrant (Piper nigrum), are chosen. The findings of the tablet evaluation parameters are placed in to the JMP software for model significance evaluations. For each of the 13 formulations, the answers are reported in terms of hardness, friability and disintegrant. Table 6 contains the response data, as well as responses surfaces for hardness (Figure 1), Friability (Figure 2) and disintegration time (Figure 3). The hardness will grow as the binder content increases, while the friability will increase. Increased disintegrant concentration will shorten the disintegration period and lower the hardness level. As a result, the optimal binder and disintegrant ratio is required. The optimum ratio is shown in the formula CT 07, CT 09 and CT 11. Because the p value was less than 0.1%, the DoE surface response of all Y1, Y2 and Y3 revealed that the model was significant. Formulation CT 07, CT 09 and CT 11 meets all of the requirements better than the other 13 formulation, as shown by the overlay plot of the trials, and therefore evaluated for optimization.
Figure 1: Hardness responses.
Response Hardness (kg/cm2)
Actual by Predicted Plot 3D Surface
Independent Variables Response Grid Slider 2.89
|
X |
Y |
|
Value |
|
|---|---|---|---|---|
|
(x) |
() |
A: Teminalia bellirica (mg) (2,10) |
6 |
|
|
() |
(x) |
B: Piper nigrum (mg)(1,3) |
2 |
Figure 2: Friability responses.
Response Friability (%)
Actual by Predicted Plot 3D Surface
Independent Variables
Response Grid Slider 0.5837
|
X |
Y |
|
Value |
|
|
|---|---|---|---|---|---|
|
(x) |
() |
A: Teminalia bellerica (mg)(2,10) |
6 |
|
|
|
() |
(x) |
B: Piper nigrum (mg)(1,3) |
2 |
|
Figure 3: Disintegration responses.
Response Disintegration time (min.sec)
Actual by Predicted Plot 3D Surface
Independent Variables
Response Grid Slider 11.125
|
X |
Y |
|
Value |
|
|
|---|---|---|---|---|---|
|
(x) |
() |
A : Teminalia bellerica (mg)(2,10) |
6 |
|
|
|
() |
(x) |
B : Piper nigrum (mg)(1,3) |
2 |
|
Figure 4: Prediction Profiler of optimization of chewable tablet.
Figure 5: Over plot of responses.
Contour Profile
Figure 6: Anti-microbial activities results.
Table 6: Surface responses by DoE results.
|
Response |
RMSE |
R Sq |
P-Value |
Model |
|
Y1 |
0.3179 |
0.67 |
0.0990 |
Significant |
|
Y2 |
0.054 |
0.37 |
0.5617 |
Significant |
|
Y3 |
0.5546 |
0.64 |
0.1337 |
Significant |
Table 7: Accelerated Stability result for chewable tablets.
|
Parameter |
Initial |
Room temperature |
Accelerated temperature |
||||
|
25°C±2°C /RH 60±5% |
40°C±2°C /RH 60±5% |
||||||
|
7th day |
15th day |
30th day |
7th day |
15th day |
30th day |
||
|
Hardness(kg/cm²) |
2.47 |
2.47 |
2.51 |
2.49 |
2.46 |
2.45 |
2.44 |
|
Friability (%) |
0.59 |
0.59 |
0.58 |
0.59 |
0.59 |
0.60 |
0.58 |
|
Disintegration time (min.sec) |
11.13 |
11.13 |
11.14 |
11.14 |
11.18 |
11.24 |
11.17 |
Accelerated Stability Testing
The stability analysis for formulation CT 07, CT 09 and CT 11 was undertaken for 30 days at two distinct temperatures, room temperature (25 ℃± 2℃/RH 60%) and accelerated temperature (40℃± 2℃ / RH 75%) with the results (Table 7) indicating that the poly-herbal chewable tablets were stable.
Table 8: Anti-microbial activity results.
|
Sr. No |
Sample solution |
Concentration in well (µl) |
Zone of inhibition (mm) |
|
1 |
Ethanol |
1000 |
- |
|
2 |
Inula racemosa |
1000 |
30 |
|
3 |
Pistacia integgerima |
1000 |
20 |
|
4 |
Myrica nagi |
1000 |
16 |
|
5 |
Solanum indicum |
1000 |
2 |
|
6 |
Acacia catechu |
1000 |
4 |
|
7 |
Clerodendrum serratum |
1000 |
10 |
|
8 |
Terminalia bellerica |
1000 |
24 |
|
9 |
Piper nigrum |
1000 |
10 |
|
10 |
Ciprofloxacin |
5 |
40 |
|
11 |
Optimized chewable tablet |
1000 |
20 |
Anti-microbial Activity Study
Agar diffusion was used to assess the anti-microbial activity of plant extracts with ethanol and polyherbal chewable tablet against Streptococcus aureus. The diameter of the inhibition zone was measured to determined anti-microbial activity. The results of the anti-microbial activity assessment were recorded. The zone of inhibition was 20mm in a polyherbal chewable tablet that had been optimised. As a result, the antimicrobial activity of the optimised polyherbal chewable tablets has been demonstrated.
ACKNOWLEDGEMENT
The author’s kindly acknowledge KLE College of Pharmacy Belagavi for the support
FINANCIAL SUPPORT &SPONSORSHIP
No financial support & sponsorship
CONFLICTS INTERESTS
No conflicts of interests
REFERENCES
- Maurya HA, Kumar TI. (2019). Formulation, standardization, and evaluation of polyherbal dispersible tablet. Int J App Pharm. 11(1):158-167.
- Shah R, Bansal A, Singhi SC. (2011). Approach to a child with sore throat. Ind J Pediatr. 78(10):1268-1272.
- Mishra KK, Tasneem K, Jain V, Mahajan SC. (2017). Formulation and evaluation of herbal lozenges. J Drug Del Ther. 7(7):87-90.
- Palakurthi SS, Jakka D, Singh H, Bollavaram S, Sininandini B, Pinnamraju DN, Konde A. (2020). Preparation and Evaluation of Chewable Tablets of Syzygium cumini Seed Powder. J Drug Del Ther. 10(3):58-64.
- Ghorpade P, Siddiqui A, Patil MJ, Rub RA. (2012). Pharmacognostic and phytochemical evaluation of Celosia argentea. Pharm J. 4(33):7-15.
- Akhtar SA, Dev PU. (2017). Formulation and evaluation of chewable multivitamin tablet. Int J Cur Pharm Res. 9(4):61-64.
- Patil NS, Salunkhe K. (2019). Formulation Development and Evaluation of Gastro Retentive Floating Tablet of Atorvastatin using Statistical Design (JMP Software). J Drug Del Ther. 9(4-A):19-25.
- Balekundri A, Shahapuri A, Patil M. (2020). Poly-herbal tablet formulation by design expert tool and in vitro anti-lipase activity. Future J Pharm Sci. 6(1):1-1.
- Qaralleh HA, Al-Limoun MO, Khlaifat A, Khleifat KM, Al-Tawarah N, Alsharafa KY, Abu-Harirah HA. (2021). Antibacterial and antibiofilm activities of a traditional herbal formula against respiratory infection causing bacteria. arXiv preprint arXiv:2102.04301.
 Abstract
Abstract  PDF
PDF